NMT Professor Examining How Titanium Implants Promote Growth of Bone
October 19, 2020
Dr. Rebecca Reiss co-authors new paper with implications on improving longevity of medical devices
SOCORRO, N.M. – Biology professor Dr. Rebecca Reiss has published new research that sheds light on how titanium implants interact with human tissue, potentially paving the way for a new generation of medical inserts that last longer.
Titanium routinely is used successfully in dental implants, bone screws, hip replacements, and other medical devices that are in contact with bone. However, scientists don’t fully understand why this metal – which otherwise isn’t found in the biological world – is so compatible with living cells and seamlessly fuse with bone.
 Reiss (pictured at right) and her collaborator Dr. Terry Lowe of Colorado School of
Mines, are investigating properties of titanium that may help improve on this compatibility.
Scientists first discovered that titanium readily integrates with bone tissue in the
early 1950s.
Reiss (pictured at right) and her collaborator Dr. Terry Lowe of Colorado School of
Mines, are investigating properties of titanium that may help improve on this compatibility.
Scientists first discovered that titanium readily integrates with bone tissue in the
early 1950s.
The main thrust of this new research is that the smaller grain size of the titanium used in medical implants promotes growth of cells. They are specifically looking at how preosteoblast cells – or bone forming cells – grow when in contact with titanium.
“We’ve found that we get more cells that are bigger and healthier on ultra-fine grain titanium versus coarse grain” Reiss said.
Reiss is a geneticist and professor emerita in the Department of Biology at NMT. Lowe was formerly a professor in the NMT Materials Engineering Department before moving to Colorado School of Mines. They have fostered a transdisciplinary research effort that resulted in the elucidation of the pathways by which cells respond to abiotic signals by comparing the response of cells to a new nanostructured variant of titanium with conventional titanium.
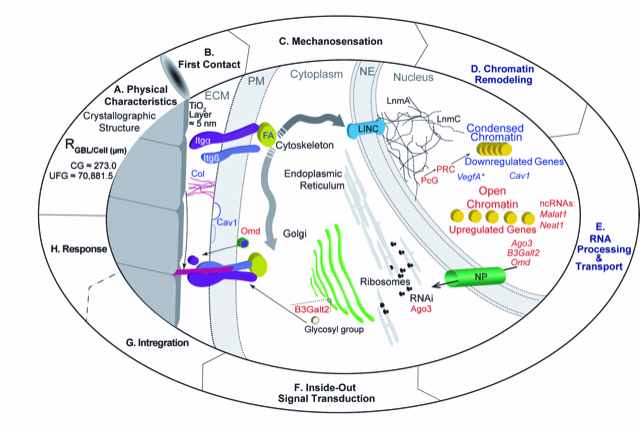
They published a paper in April 2020 titled "Effect of surface grain boundary density on preosteoblast proliferation on titanium". That publication compares the physical characteristics of the titanium substrates and confirms that cells grow faster on the nanostructured substrate. The latest article in PLOS ONE focuses on the biology of titanium implants. Published in September 2020, the paper is titled "Bio-activating ultrafine grain titanium: RNA sequencing reveals enhanced mechano- activation of osteoconduction on nanostructured substrates." This paper provides a snapshot of the changes that occur in the nucleus as a result of contact with the nanostructured titanium.
 Other co-authors on this project include Oleg Makhnin, mathematics professor at NM
Tech, and Johnny Sena from the National Center for Genome Resources. Three students
also worked on this project and are co-authors: NMT master’s graduates Patrick Illescas
and Melanie Connick, and Dr. Casey Davis, who earned her Ph.D. at Colorado School
of Mines in 2019.
Other co-authors on this project include Oleg Makhnin, mathematics professor at NM
Tech, and Johnny Sena from the National Center for Genome Resources. Three students
also worked on this project and are co-authors: NMT master’s graduates Patrick Illescas
and Melanie Connick, and Dr. Casey Davis, who earned her Ph.D. at Colorado School
of Mines in 2019.
It has been over a decade since nanotechnology was applied to bulk titanium to improve its compatibility with human cells by changing its nano-size scale crystallographic structure and reducing the size of its constituent crystals. The paper includes a model of how the nanoscale features improve compatibility with bone tissue. This sets the stage to use these features to further decipher how cells respond to the environment.
The techniques used to change the crystallographic structure are different from those used to produce nanoparticles or titanium oxides that are commonly used in food and sunscreens. Solid (bulk) titanium is inert, resistant to corrosion, and is too big to enter cells so that it acts mainly through how its features can communicate to cells.
With a pilot project grant from the National Center for Genome Resources in Santa Fe, Reiss and Lowe applied Next-Generation DNA Sequencing to compare gene expression in cells encountering nanostructured bulk titanium with a reduced crystal structure to cells growing on standard coarse grain titanium, as is currently used in implants.
This research identifies the genes that bulk titanium activates even without entering cells, a process the authors call "bio-activation.” The results will lead to a new generation of implantable materials. The researchers are now turning their attention to understanding and improving other biological effects of metals and nanostructures, including virucidal properties of other metals.
– NMT –